Key practice points
- Use the lowest potency
topical corticosteroid needed to control the patient’s symptoms
- Be clear when prescribing
where each product should, and should not, be used; avoid the term “use sparingly”
- Check that patients and
caregivers can identify a flare and are able to respond with appropriate treatment
- For patients with persistent
eczema, short treatment “bursts”, e.g. three to five days, with higher potency corticosteroids may be preferable
to longer courses of treatment with less potent corticosteroids; topical corticosteroids should be stepped down, e.g. from
potent or moderate potency to mild potency, as the patient’s symptoms resolve
- Include descriptions of
potency in the prescription so that it is printed on the medicine label to avoid confusion
For general information on the treatment of childhood eczema, see the companion article:
“Childhood eczema: improving adherence to treatment basics”.
This is a revision of a previously published article. What’s new for this update:
- Changes to topical calcineurin
inhibitor funding status:
- Topical pimecrolimus is now funded with Special Authority approval for treatment of eczema affecting
the eyelid if topical corticosteroids cannot be used
- Tacrolimus ointment is now an approved medicine in New Zealand for people aged ≥ 16
years and will be funded with Special Authority approval from October, 2021, for eczema affecting the face if topical
corticosteroids cannot be used
- Table of topical corticosteroids
has been updated to reflect currently funded brands
Topical corticosteroids are one of the key medicines used in the management of childhood eczema. However, adherence is typically poor,
often due to “corticosteroid phobia”. Common themes contribute to the reluctance of caregivers to use topical corticosteroids (Table
1). Addressing these concerns may improve treatment adherence and patient outcomes.2
Table 1: Caregiver misconceptions and concerns associated with the use of topical corticosteroids for eczema in children
and evidence-based responses.3–6
| Misconception or concern |
What does the evidence say? |
| Topical corticosteroids should only be used for severe symptoms |
Topical corticosteroids can and should be used for all severities of eczema, including mild symptoms
read more
Products have a range of potencies to treat patients with differing symptom severity. Treatment should be with the mildest topical
corticosteroid which is able to resolve the inflammation within a short period of time so that the patient is able to have days without
using topical corticosteroids. Different potencies are required for different parts of the body depending on the thickness of the
stratum corneum. |
| Regular use of topical corticosteroids causes adverse effects such as skin thinning |
Topical corticosteroids are unlikely to cause skin thinning or other long-term harm to children if used appropriately
read more
Skin thinning is one of the most frequently cited concerns reported by patients and caregivers, however, is very unlikely to occur if patients
and caregivers use topical corticosteroids appropriately.2, 7, 8 This consensus is based on research and clinical experience from Australia and
New Zealand, including evaluations of children treated with potent corticosteroids.7 Skin thinning is more likely to occur in adults, or in
areas with a thinner stratum corneum, such as the face and groin.2 |
| The percentage of a topical corticosteroid is its strength |
The percentage value of different formulations of topical corticosteroids does not indicate their potency, e.g.
hydrocortisone 1% is a weaker formulation than hydrocortisone butyrate 0.1%. |
| Corticosteroids are confused with anabolic steroids |
Clarify the meaning of the word “steroid”
read more
Inform patients and caregivers that the label “steroids” is a classification used for a wide group of hormones
and medicines with different functions, including corticosteroids and anabolic steroids. |
| Topical corticosteroids should not be applied to broken skin |
The consensus of paediatric dermatologists in Australia and New Zealand is that topical corticosteroids can be
applied to areas of eczema with broken skin5
read more
This recommendation possibly arose as topical corticosteroid absorption will be greater through broken skin. However, this can prevent patients
having topical corticosteroids applied to areas of active eczema particularly when severely inflamed or excoriated. All skin with an active eczema
flare will have reduced barrier function, and the best way to address this is through appropriate use of topical corticosteroids.9 |
| Topical corticosteroids are not “natural” |
Corticosteroids mimic the effects of hormones produced by the adrenal glands, despite being “man-made” |
* For further information on symptom severity and recommended treatment escalation, see:
www.bpac.org.nz/BPJ/2015/April/eczema.aspx
Avoid “use sparingly”: encourage appropriate use
Advising patients to “use topical corticosteroids sparingly” creates confusion; patients and caregivers are prescribed
a medicine but simultaneously warned against using it.5
This advice may result in corticosteroids only being used when symptoms are severe, leading to inadequate use and poor
symptom control. Caregivers should instead be encouraged to “use corticosteroids appropriately”, which will maximise the
benefits of use and minimise adverse effects.
Patients/caregivers should know:9
- Which corticosteroid to apply, i.e. using the right potency and formulation
- Where on the body to apply it
- When to apply it, i.e. when to start treatment and how long to use it for
- How much to apply
Arrange to review the patient within two to four weeks of prescribing topical corticosteroids. This gives an opportunity to assess
their response to treatment and reinforce education as well as allowing the patient and caregiver to focus on treating the
eczema rather than watching for adverse effects.
There are a range of fully funded or partly funded topical corticosteroids available to prescribe for children with eczema
(Table 2).10, 11 In addition, some topical corticosteroids are available over-the-counter
without a prescription, including:10
- Pharmacy only medicine – hydrocortisone 0.5%
- Pharmacist only medicines – hydrocortisone 1% and clobetasone butyrate 0.05%, which are also available fully funded
or partly funded on prescription, respectively
Consider the consistency of the product required:1, 12
- Creams, lotions or gels are useful for large areas of skin
- Lotions, solutions or gels are useful for the scalp or other areas with hair
- Ointments are useful for very dry skin and skin with thick scale
Key points when selecting the potency of topical corticosteroids include:2, 5, 7–9,
13
- Use the lowest potency corticosteroid needed to control symptoms, e.g. hydrocortisone 1% daily or twice daily for mild
eczema. However, be prepared to increase potency, particularly for eczema on the trunk and limbs, if a mild topical corticosteroid
is not working.
- For patients with persistent eczema, short “bursts” with higher potency corticosteroids, e.g. betamethasone valerate
0.1% twice daily for three to five days, may be preferable to longer courses of treatment with less potent corticosteroids.
Betamethasone valerate 0.1% twice daily for three days is as effective as hydrocortisone 1% twice daily for seven days.
Patients can be treated with a higher potency corticosteroid initially to gain control of symptoms and then stepped down
to a less potent formulation, e.g. hydrocortisone 1%.
- This results in quicker resolution of symptoms and shorter treatment duration
- If patients are switched to higher potency corticosteroids ensure they understand that the treatment period is shorter
- If a lower potency of corticosteroid is needed, prescribe a weaker corticosteroid rather than diluting a more potent
formulation
- Diluting topical corticosteroids with emollients does not result in a less potent medicine. Potency is related to
the affinity of the particular corticosteroid molecule to the receptor.
- Include corticosteroid potency on medicine labels
- Patients and caregivers may believe that the percentage of a topical corticosteroid determines it’s strength, e.g.
that hydrocortisone 1% is stronger than hydrocortisone butyrate 0.1%, without realising that different corticosteroids
have differing potencies.4 Labelling a topical corticosteroid as mild, moderate, potent or very potent (Table
2) or similar terms that will be clear to patients, e.g. low, medium, strong, very strong, on medicine labels, avoids
confusion and the risk of inappropriate use.
- Very potent topical corticosteroids, i.e. betamethasone dipropionate 0.05% (in propylene glycol base) and clobetasol
propionate 0.05%, should not be initiated in children without prior discussion with a dermatologist
Provide a written plan for the patient and caregiver to take home. This can help to remind them which topical
corticosteroid to apply where. For an example, see: www.starship.org.nz/for-health-professionals/new-zealand-child-and-youth-clinical-networks/child-and-youth-eczema-clinical-network/family-information-and-handouts/
Table 2: Prescription only topical corticosteroid potency and currently funded formulations, sizes and brands, as of July, 2021.10, 11
Check the NZF or Pharmaceutical Schedule for up to date information on funding status.
| Potency |
Active ingredient |
| Formulations available |
|
|
|
Lotions or liquids
Useful for large areas of skin, the scalp or areas with hair
|
Cream
Useful for large areas of skin
|
Ointment
Useful for skin with thick scale
|
| Prescription only medicines |
| Mild |
Hydrocortisone 1% |
|
|
100 g, 500 g
Hydrocortisone PSM
|
|
Moderate
(2 - 25 × as potent as hydrocortisone)
|
Clobetasone butyrate 0.05% |
|
|
30 g
Eumovate |
|
| Triamcinolone acetonide 0.02% |
|
|
100 g
Aristocort
|
100 g
Aristocort |
Potent‡
(100 - 150 × as potent as hydrocortisone)
|
Betamethasone dipropionate 0.05%* † |
|
|
15 g, 50 g
Diprosone
|
15 g, 50 g
Diprosone |
| Betamethasone valerate 0.1%† |
|
Lotion 50 mL
Betnovate
Application 100 mL
Beta Scalp Application
|
50 g
Beta
|
50 g
Beta |
| Diflucortolone valerate 0.1% |
|
|
|
50 g
Nerisone |
| Hydrocortisone butyrate 0.1% |
|
Scalp Lotion 100 mL
Locoid Scalp
Topical emulsion 100 mL
Locoid Crelo
|
100 g
Locoid Lipocream
|
100 g
Locoid |
| Methylprednisolone aceponate 0.1% |
|
|
15 g
Advantan
|
15 g
Advantan |
| Mometasone furoate 0.1% |
|
Lotion 30 mL
Elocon
|
15 g, 50 g
Elocon Alcohol free
|
15 g, 50 g
Elocon |
Very potent‡
(up to 600 × as potent as hydrocortisone)
|
Betamethasone dipropionate 0.05%* † |
|
|
|
30 g
Diprosone OV |
| Clobetasol propionate 0.05%†
|
|
Application 30 mL
Dermol
|
30 g
Dermol |
30 g
Dermol |
* Betamethasone dipropionate is available as a potent formulation (Diprosone) or modified formulation with increased
potency (Diprosone OV; very potent), both containing 0.05% active ingredient
† Not approved for use in children aged under 12 months10
‡ Note that the face, flexural areas, genitals and the groin are more prone to irritation and skin atrophy than other sites; treatment of
these areas is usually limited to mild or moderate potency topical corticosteroids8, 13
Fully funded
Partly funded
Where on the body should they be applied?
Topical corticosteroids should only be applied on actively affected areas of eczema in an appropriate amount (see: “How
much should be used?”).10 When prescribing, be clear where each product should be used, e.g. lower potency
topical corticosteroids for the face, and specify the treatment duration and any areas of the body where use of the corticosteroid
would be inappropriate (Figure 1). For example:7, 9
Methylprednisolone aceponate 0.1% cream
Potent (strong) corticosteroid - apply once daily to eczema on the limbs and
trunk until the flare has cleared. Seek medical attention if symptoms persist after seven days.
Mitte 15 g and 2 repeats
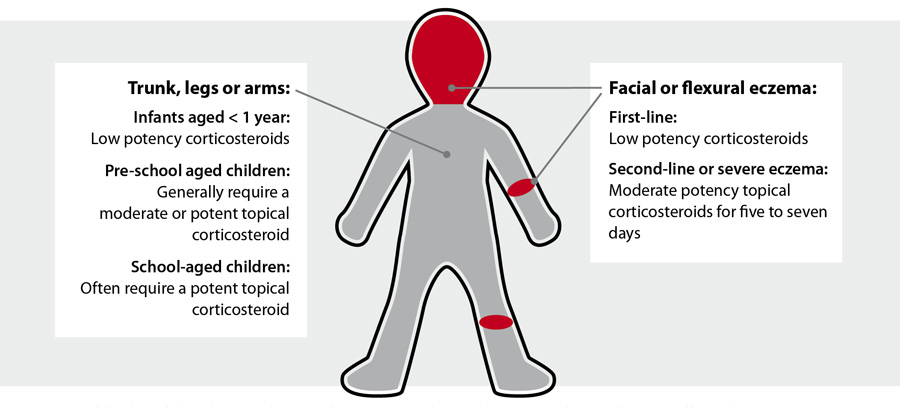
Figure 1: “Rules of thumb” to guide topical corticosteroid use, from www.bpac.org.nz/BPJ/2016/February/eczema.aspx
Caution is required when applying topical corticosteroids to the face, periorbital or perioral regions and flexural or groin areas
The face, flexural and groin areas are more susceptible to adverse effects such as striae or skin atrophy
and systemic absorption is increased in these areas compared to other sites.2 For children with eczema affecting
the face, use mild potency or short courses of moderate potency topical corticosteroids.13 In flexural or groin
areas moderate or potent topical corticosteroids should be used only for short periods, e.g. up to seven days.13
In periorbital regions potent or very potent topical corticosteroids should not be used.
In perioral regions the use of even mild topical formulations has been associated with the development
of periorificial dermatitis or “steroid rosacea”.7 Ongoing use of topical corticosteroids may aggravate these
conditions.7
Topical calcineurin inhibitors are an alternative treatment if the use of topical corticosteroids
is contraindicated or not appropriate.13, 14 There are two topical calcineurin inhibitors approved for use in
New Zealand:11, 14
- Pimecrolimus is funded with Special Authority approval for treatment of eczema affecting the eye lid if topical corticosteroids
cannot be used
- Tacrolimus ointment is approved for use in people aged ≥ 16 years and will be funded from 1 October, 2021, with Special
Authority approval for eczema affecting the face if topical corticosteroids cannot be used
Patients may self-fund for other indications. Calcineurin inhibitors are more likely to cause a burning sensation and
pruritis than topical corticosteroids.10, 15 A possible association between topical calcineurin inhibitor use
and increased risk of lymphoma has been examined in a recent systematic review and meta-analysis.16 Analyses
did find an association, however, the overall risk is very small.16
When should they be applied?
Check that patients and caregivers understand when to initiate treatment with topical corticosteroids and
when treatment should be stepped down or withdrawn:2, 8, 13
- Emollient use should continue during flares
- Topical corticosteroids should only be applied to areas of active eczema, unless during “weekend treatment” (see: “How
long should they be applied for?”)
- Initially, once daily application of topical corticosteroids is often sufficient; no more than twice daily. As symptoms
improve treatment can be stepped down by either applying a lower potency corticosteroid with the same frequency, or the
same potency corticosteroid applied less frequently.
How long should they be applied for?
Topical steroids should generally be effective in clearing inflammation so that long-term treatment is primarily
with emollients.
- Flares should typically resolve within seven to 14 days of treatment.8 If treatment is not effective, check
adherence, consider if the treatment should be changed or re-consider the diagnosis.8
- For patients in whom treatment is effective but they have frequent flares, “weekend treatment”, also known as maintenance
therapy, should be considered. This consists of applying topical corticosteroids for two days a week during remission.13
- A study involving patients using “weekend treatment” of betamethasone dipropionate (0.05%) showed that 74% of participants
maintained remission for 12-weeks and developed no adverse effects like skin atrophy or Cushing’s syndrome (see: “The
adverse effects of topical corticosteroids are mild and reversible”).1 Since this study, large-multicentre
studies have achieved similar results.1
How much should be used?
Calculate how much topical corticosteroid to prescribe and if possible, provide an indication of when a repeat prescription
is likely to be required. Caregivers can use fingertip units (FTU) to guide the amount of topical corticosteroid to apply
(Table 3 and Figure 2). A fingertip unit is the amount of product that covers the
tip of an adult’s index finger to the distal skin crease from a standard 5 mm tube.10 This is a sufficient quantity
for an area of skin equal to the palms of two adult hands. One FTU is approximately 0.5 g.10
For example, a child aged five years with eczema mainly affecting one arm and hand will require approximately four FTU
of topical corticosteroid per application (Table 3). If this is applied once daily during flares, and
flares last approximately seven days in total during a month, this would equate to:
4 × 0.5 g × once daily × seven days = approximately 14 g
Usage may vary depending on the extent of flares, how quickly they resolve, whether topical corticosteroid use is tapered
or stepped down, and whether patients are also using topical corticosteroids during “weekend treatment”.
Table 3: Approximate number of adult fingertip units (FTU) of corticosteroid needed per application for children with eczema.1, 10 *
|
3 – 6 months |
1 – 2 years |
3 – 5 years |
6 – 10 years |
11 – 18 years |
| One entire arm and hand |
1 |
1.5 |
2 |
2.5 |
4 |
| One entire leg and foot |
1.5 |
2 |
3 |
4.5 |
8 |
| Torso (front) |
1 |
2 |
3 |
3.5 |
7 |
| Back and buttocks |
1.5 |
3 |
3.5 |
5 |
7 |
| Face and neck |
1 |
1.5 |
1.5 |
2 |
2.5 |
* Note that these values are a guide and will be influenced by the size of the child
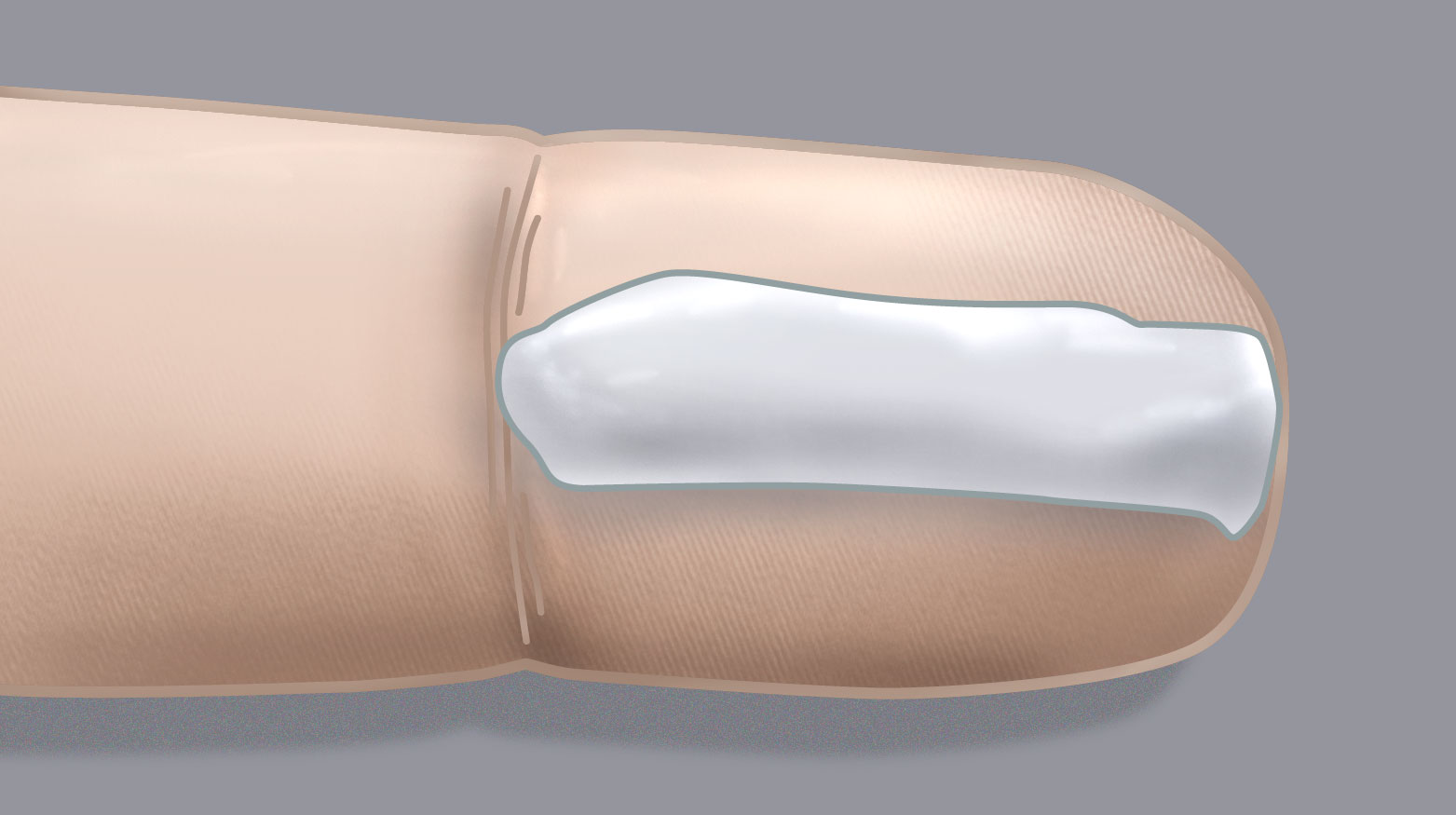
Figure 2. Fingertip unit
Shortly after application of a topical corticosteroid some patients may experience local irritation or a change in skin
colour caused by corticosteroid-induced vasoconstriction.2 Hypopigmentation typically clears when the topical
corticosteroid is stopped.7 Changes in pigmentation usually occur due to the eczema itself or another dermatological
condition, e.g. pityriasis alba.7, 17
There is little evidence as to what percentage of a topical corticosteroid dose is absorbed systemically. Studies
investigating systemic effects do not measure how much of the corticosteroid is in the blood, but instead
focus on measuring cortisol as a marker of hypothalamic-pituitary-adrenal (HPA) axis suppression. After
a few weeks’ treatment with potent or very potent
topical corticosteroids temporary HPA axis suppression does occur. However,
this resolves upon cessation of the topical corticosteroid, without the need for dose tapering.7 HPA axis
suppression is more likely when topical corticosteroids are applied under occlusion, e.g. with wet wraps
as greater systemic absorption occurs.8,
13
Inappropriate or prolonged use may cause more serious adverse effects
More serious adverse effects include clinically significant HPA axis suppression, skin atrophy or striae or withdrawal
symptoms upon stopping the corticosteroid, such as erythema and aggravation of cutaneous symptoms.1, 2, 13 These
are rarely seen with normal prescribing patterns.
The risk of these adverse effects is increased:7, 8
- With a higher potency of corticosteroid
- With application to a greater area of skin or a larger quantity of application
- When corticosteroids are applied under occlusion or to flexural or groin areas, which increases absorption
- If patients are also taking oral or high-dose inhaled corticosteroids
- When potent topical corticosteroids are applied to striae-prone areas, e.g. axillae or groin areas, during growth phases
of puberty
If patients request repeat prescriptions earlier than expected consider whether they may be using a topical corticosteroid
inappropriately; case reports of adverse effects typically involve patients who have used the product for longer than it
was prescribed for.2
Ask patients to bring their tubes of topical corticosteroids with them to appointments so you can more accurately
assess the quantities used
Patient information on the use of topical corticosteroids is available from:





